

The higher the value, the larger risk there is to supply. The percentage of an element produced in the top producing country. Low = substitution is possible with little or no economic and/or performance impact Medium = substitution is possible but there may be an economic and/or performance impact High = substitution not possible or very difficult. The availability of suitable substitutes for a given commodity. A higher recycling rate may reduce risk to supply. The percentage of a commodity which is recycled. The number of atoms of the element per 1 million atoms of the Earth’s crust. This is calculated by combining the scores for crustal abundance, reserve distribution, production concentration, substitutability, recycling rate and political stability scores. The Chemical Abstracts Service registry number is a unique identifier of a particular chemical, designed to prevent confusion arising from different languages and naming systems.ĭata for this section been provided by the British Geological Survey.Īn integrated supply risk index from 1 (very low risk) to 10 (very high risk).
Where more than one isotope exists, the value given is the abundance weighted average.Ītoms of the same element with different numbers of neutrons.
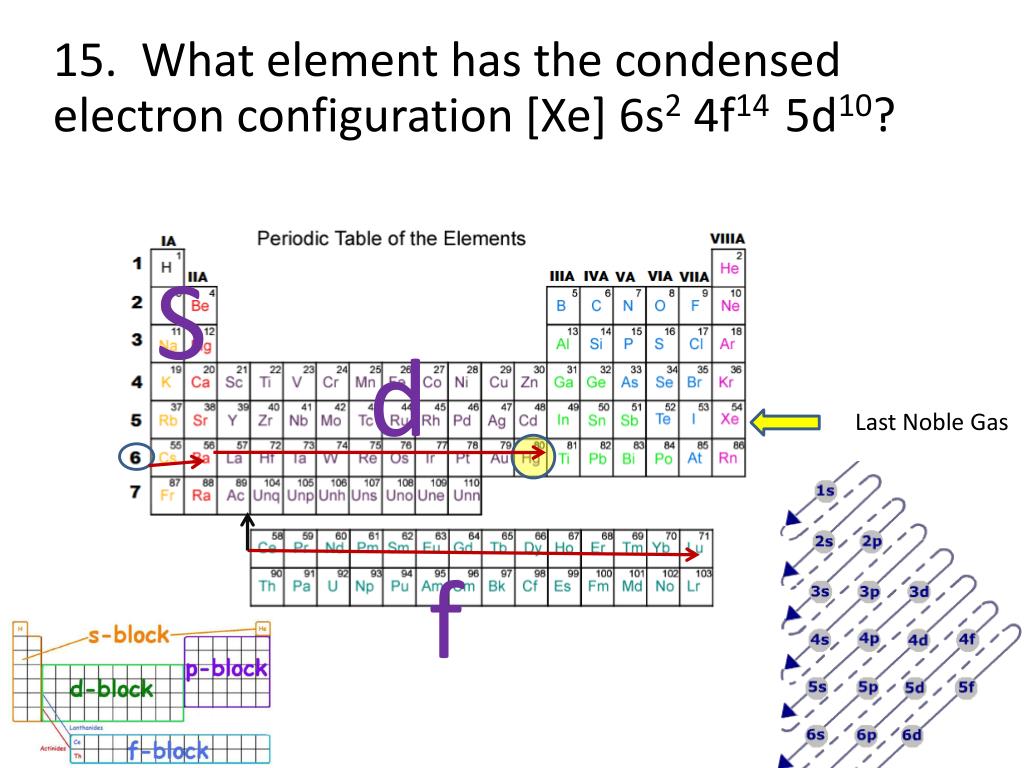
This is approximately the sum of the number of protons and neutrons in the nucleus. The mass of an atom relative to that of carbon-12. The transition of a substance directly from the solid to the gas phase without passing through a liquid phase.ĭensity is the mass of a substance that would fill 1 cm 3 at room temperature. The temperature at which the liquid–gas phase change occurs. The temperature at which the solid–liquid phase change occurs. The arrangements of electrons above the last (closed shell) noble gas. These blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). The atomic number of each element increases by one, reading from left to right.Įlements are organised into blocks by the orbital type in which the outer electrons are found. Members of a group typically have similar properties and electron configurations in their outer shell.Ī horizontal row in the periodic table. This makes it easier to study and model how atoms will interact to form chemical bonds.A vertical column in the periodic table. The electron configuration is of great help to scientists as it provides an easy method for writing and communicating how electrons are arranged around the nucleus of an atom. It is also used in the prediction of the behavior of atomic and molecular systems, such as in the development of quantum mechanical models and in the study of electronic structure and bonding. The electron configuration of a neutral carbon atom is 1s 2 2s 2 2p 2, indicating that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and two electrons in the 2p orbital.Įlectron configuration is important because it determines the chemical properties of an atom or molecule, including its reactivity and stability. In general, the electron configuration of an atom or molecule can be represented by writing the orbitals in order of increasing energy, starting with the lowest energy orbital, and indicating the number of electrons in each orbital using the electron-dot notation.įor example, the electron configuration of a neutral helium atom is 1s 2, indicating that there are two electrons in the 1s orbital. It indicates the distribution of electrons among the various atomic orbitals, which are the regions around the nucleus where electrons are likely to be found. General and abbreviated electron configurationĮlectron configuration is a way of describing the arrangement of electrons in an atom or molecule.This result will consist of the following data: Then you just have to press the “ Solve” button and a box with the result will automatically open.In case of selecting ion, you must indicate its charge, using the + or – signs followed by the number For example, if we want to obtain the electronic configuration of the sulfide ion (S -2), we must select the sulfur element, select the ion option and then enter -2 in the box corresponding to the charge. Indicate if you want to obtain the electronic configuration of the selected element or of an ion.To use the Electronic Configuration Calculator you must follow these steps: Which will allow you to quickly know the electronic configuration of any element in its general and abbreviated form. To help all those students or professionals related to the field of chemistry, we have created the Electronic Configuration Calculator.


 0 kommentar(er)
0 kommentar(er)
